How to apply for coronavirus vaccine trial
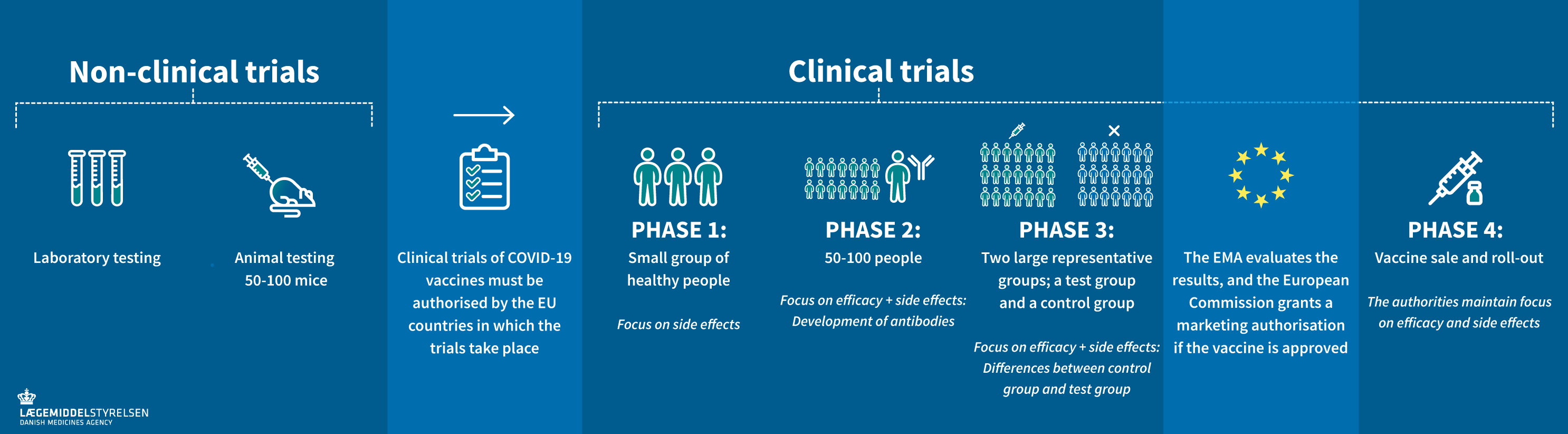
The three c oronavirus vaccines currently approved for use in the UK through temporary authorisations have been through all the normal stages of vaccine testing, including animal and human studies. In Decemberthe Pfizer and the Oxford-AstraZeneca research teams released their analyses of phase three safety and efficacy data. Moderna released theirs in February For Pfizer this was a peer-reviewed article in the New England Journal of Medicine including efficacy data from 43, participants including placebo groupwith two month safety data from 37, participants.
For Oxford-AstraZeneca, this was a peer reviewed article in The Lancetincluding interim analysis data from around 11, participants although 23, participants were included in the wider trial from April-November The second image included in the Facebook post, is a picture of a genuine page on the UK government website. This is correct, but needs more context.

We wrote about this in However, by March there was awareness that the mortality rates were lower than initially feared, there was improved clinical awareness, and an accurate test for SARS-CoV-2 the virus that causes Covid was available. Emergency use authorization EUA in the U. When the pandemic is over, the EUA will cease and vaccine manufacturers will need to apply for full U. No timeline on this has yet been given here. The UK, meanwhile, has a similar mechanism herehere. They both use a modified and weakened version of a harmless adenovirus to deliver instructions to cells to make coronavirus spike proteins.
In addition, infertility is not known to occur as a result of natural COVID disease, further demonstrating that immune responses to the virus, whether induced by infection or a vaccine, are not a cause of infertility. Reports on social media how to apply for coronavirus vaccine trial falsely asserted that the vaccine could cause infertility in women and the FDA is concerned that this misinformation may cause women to avoid vaccination to prevent COVID, which is a potentially serious and life-threatening disease. The symptoms of COVID vary and are unpredictable; many people have no symptoms or only mild disease, while some have severe respiratory disease including pneumonia and acute respiratory distress syndrome ARDSleading to multi-organ failure and death. Comirnaty how to apply for coronavirus vaccine trial a mRNA vaccine. After a person is vaccinated, their body produces copies of the spike protein, which does not cause disease, and triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV Contrary to false reports on social media, this protein is not the same as any involved in the formation of the placenta.
Registro de inscripción para ensayos de vacunas contra COVID-19
These participants were followed for safety outcomes. The EUA remains in effect for how to apply for coronavirus vaccine trial two dose primary series in individuals 12 years of age and older and as a third primary dose for individuals 12 years of age and older who have been determined to have certain kinds of immunocompromise.
Additionally, the FDA authorized the vaccine for emergency use to allow for a single booster dose administered at least 6 months after completion of the vaccination primary series in certain populations. The trial, which will be conducted at U. Fauci, M. This scientifically rigorous, randomized, placebo-controlled trial is designed to determine if the vaccine can prevent COVID and for how long such protection may last. The vaccine efficacy trial is the first to be implemented under Operation Warp Speed, a multi-agency collaboration led by HHS that aims to accelerate the development, https://ampeblumenau.com.br/wp-content/uploads/2020/02/archive/social/how-to-use-linktree-on-instagram.php and distribution of medical countermeasures for COVID Collins, M.
Investigators will use public health data and incidence trajectory modeling check this out identify sustained high-incidence areas and emerging hot zones, so sites near these locations can be prioritized for enrollment. A Phase 1 clinical trial found the candidate vaccine to be safe, generally well-tolerated and able to induce antibodies with high levels of virus-neutralizing activity.
How to apply for coronavirus vaccine trial -
Who is eligible to be a volunteer?Current coronavirus vaccine studies
The trial is open to healthy individuals aged 18 years and older among both sexes, including: Those who are at high risk of novel coronavirus infection, "defined as adults whose locations or circumstances put them at appreciable risk of exposure," to the virus, the government website explains. Healthy adults with pre-existing medical conditions who are in stable condition are also eligible to participate. Female volunteers of childbearing potential may be enrolled in the study if the participants meet all of the following criteria: Has a negative pregnancy test at screening and on the day of the first dose of the vaccine.
Has practiced adequate contraception or has abstained from all activities that could result in pregnancy for at least 28 days prior to the first dose. Has agreed to continue https://ampeblumenau.com.br/wp-content/uploads/2020/02/archive/shopping/how-much-is-a-large-regular-coffee-at-starbucks.php contraception for three months following the second dose of the vaccine. The participant is not currently breastfeeding. how to apply for coronavirus vaccine trial

Those with a known history of novel coronavirus infection are not eligible to enroll.
How to apply for coronavirus vaccine trial - think, that
Wednesday, a new website -- coronaviruspreventionnetwork. The website will handle registration for the four large vaccine studies that are expected to start this summer and fall, and any others that follow.
Challenges in doing a very large, very complicated trial very fast Read More A vaccine developed by Moderna, a Massachusetts biotech company, is expected to be the first to be tested in a large trial. That trial was expected to begin this week, but the start date was moved to late July or early August, according to Dr.
They won't let a site start until they're absolutely click. Some could start on July 27, and others on August 8," del Rio said. Despite the delay, the Covid vaccine trials are moving at an unprecedented speed, as researchers try to accomplish in months what usually takes years. Eventually, he aims to have a total of study subjects at three Atlanta-area sites.
Menu Block - Clinical Trails
For the: How to apply for coronavirus vaccine trial
| How to apply for coronavirus vaccine trial | 789 |
| How to apply for coronavirus vaccine trial | How to join a coronavirus vaccine study. You can join any of the government-sponsored coronavirus vaccine trials by going to the CoVPN website. There, you can begin the screening process to find. Jul 09, · These later phase trials monitor safety and focus on whether the vaccine protects against becoming ill from the coronavirus.
Novak said volunteers for the Moderna trial will receive two injections Estimated Reading Time: 6 mins. |
| WHERE IS THE CLOSEST MEXICAN RESTAURANT NEAR ME | What free drink can you get at starbucks on your birthday |
To figure out if potential coronavirus vaccines actually work, researchers will soon start recruiting tens of thousands of volunteers into clinical trials. how to apply for coronavirus vaccine trial to continue reading for coronavirus vaccine trial Video Inside The Clinical Trial For Moderna’s Covid Vaccine - NBC News NOW
What level do Yokais evolve at? - Yo-kai Aradrama Message